About
Founded in 2009 by Roy Rubinfeld, MD, MA to serve as a resource for patients, their families, doctors, and researchers, CXLUSA, LLC grew to become the world’s largest crosslinking (CXL) research study group with 36 investigators across the United States. Working with top international scientific experts, this group has been instrumental in advancing less invasive treatments for those with keratoconus and other ectasias, eventually developing the treatment known as EpiSmart® to avoid the long recovery, pain, and risks of the older, more invasive form of crosslinking invented in 1998, which requires surgical removal of the front layer (epithelium) of the cornea to get the vitamin into the cornea.
World’s Largest CXL Research Study Group
The CXLUSA research study group joined together 36 internationally-recognized ophthalmologists across the United States. When CXLUSA was founded, the mission of this group of experts was to advocate for keratoconus patients, inform doctors, and improve treatments. Since then, their groundbreaking research has developed a less invasive, rapid recovery epithelium-on corneal crosslinking treatment.
CXLUSA is the world’s largest research study group that has researched and developed minimally-invasive treatment of keratoconus and post-LASIK ectasia using epithelium-on corneal crosslinking. CXLUSA has developed and shared the latest research from keratoconus clinical trials and epithelium-on CXL since 2009.
History of Epithelium-on CXL
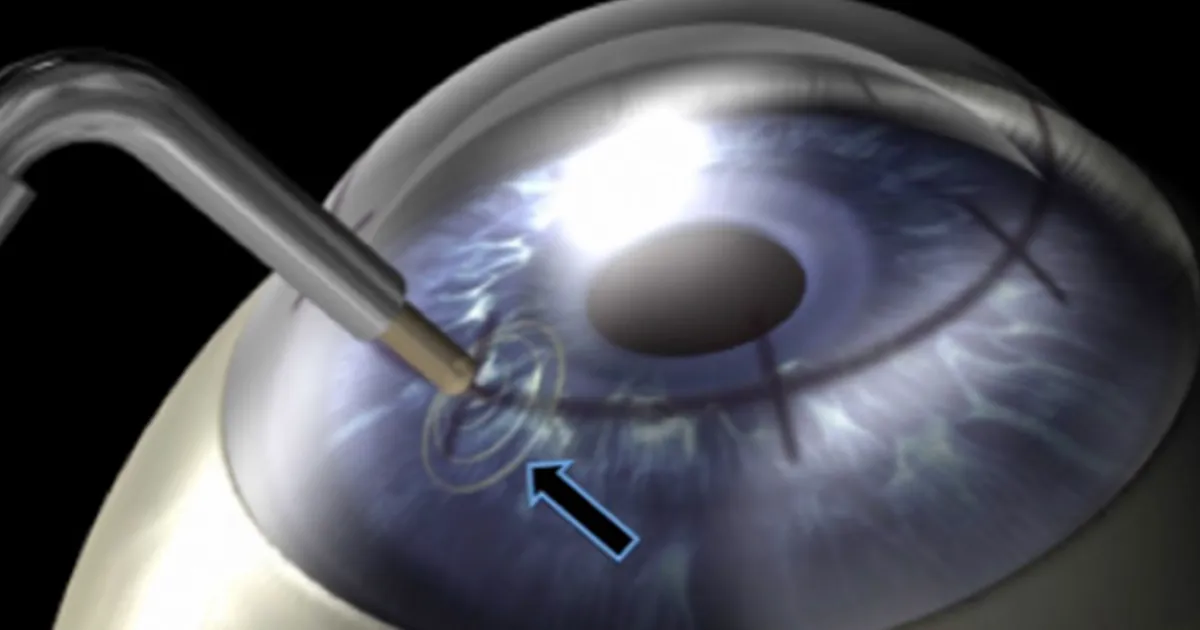
CXL in its original form has existed since 1998 as a technique to treat keratoconus and post-LASIK ectasia. These conditions are caused by a weakened, thinning cornea that becomes cone-shaped and distorts vision. CXL strengthens the cornea with vitamin B (riboflavin) and UV light. However, for the vitamin to be absorbed, the protective layer of the cornea, or the epithelium, needed to be removed. This original technique meant a long, painful recovery and risks of infection, scarring and other complications. The images below show the corneal scraping that occurred with the older “epi-off” procedure.
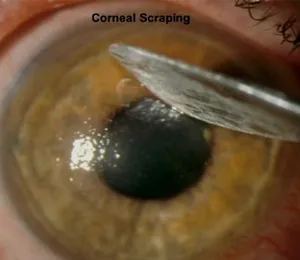
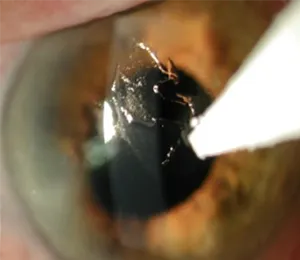
CXLUSA developed a novel vitamin formulation which has been shown in published studies to be the only system that can get the vitamin into the cornea while leaving the protective epithelium intact. This innovative, unique treatment is the only epithelium-on crosslinking shown in large published research studies to stop vision loss with a recovery period of only 1-2 days.
Timeline of CXL Innovation

Contact Us
If you have questions about epithelium-on CXL or keratoconus and other corneal ectasias, please reach out to our team. Our goal is to be a resource and make this groundbreaking research accessible.